Advertisement

- nature
- scientific reports
- articles
- article
- Article
- Open access
- Published: 23 May 2025
Treatment with hydrogen-rich water protects against thioacetamide-induced hepatic encephalopathy in rats through stabilizing liver–brain disturbance
Scientific Reports volume 15, Article number: 17901 (2025) Cite this article
- 280 Accesses
- Metrics details
Abstract
Hepatic encephalopathy (HE), a neuropsychiatric complication secondary to liver cirrhosis and hepatic failure, represents the leading cause of mortality in end-stage liver disease. While hyperammonemia remains the central pathogenic factor in HE progression, emerging evidence implicates oxidative stress, neuroinflammation, and neuronal apoptosis as critical synergistic contributors to HE pathogenesis. Hydrogen-rich water, known for its antioxidant, anti-inflammatory, and anti-apoptotic properties, has not been systematically investigated for therapeutic efficacy in HE management. In the current investigation, we successfully established a HE rat model by administering thioacetamide via intraperitoneal injection. By observing the general state and behavioral changes of the rats, detecting liver function and blood ammonia, and observing the pathological changes of liver and brain tissue, it was discussed whether hydrogen-rich water had a preventive and therapeutic effect on hepatic encephalopathy. Oxidative stress, inflammation and neuronal apoptosis were detected in plasma, prefrontal cortex and hippocampus to explore the possible mechanism of its protective effect. The results showed that hydrogen-rich water can improve the behavioral changes of the HE rats, reduce blood ammonia, reduce liver function damage, alleviate the pathological changes of liver and brain tissue, significantly inhibit the systemic and local oxidative stress and inflammation of the brain tissue of the HE rats, and reduce neuronal apoptosis. In summary, hydrogen-rich water might stabilize liver-brain disturbance in thioacetamide-induced HE rats by anti-inflammation, anti-oxidative stress and reducing neuronal apoptosis.
Similar content being viewed by others
Neuroprotective and cognitive enhancing effects of herbecetin against thioacetamide induced hepatic encephalopathy in rats via upregulation of AMPK and SIRT1 signaling pathways
Article Open access18 May 2024
Ameliorative effect of oregano (Origanum vulgare) versus silymarin in experimentally induced hepatic encephalopathy
Article Open access25 October 2022

Long-term and daily use of molecular hydrogen induces reprogramming of liver metabolism in rats by modulating NADP/NADPH redox pathways
Article Open access10 March 2022
Introduction
Hepatic encephalopathy (HE), a neuropsychiatric manifestation of hepatic insufficiency, presents with a spectrum of neurological impairments including affective disturbances, memory deficits, cognitive dysfunction, psychomotor retardation, attentional lapses, sleep-wake cycle abnormalities, and sensory processing impairments. In advanced stages, patients may progress to global neurological depression characterized by stupor or coma, severely compromising quality of life1. HE is a common complication of cirrhosis and liver failure, and about 50–70% of patients with portal hypertension and cirrhosis will eventually develop HE. HE is also the most common cause of death in patients with various liver diseases developing to the end stage, with a high mortality rate2.
The mechanism of HE is complex, and there are various hypotheses including ammonia poisoning, blood-brain barrier (BBB) abnormalities, etc. The ammonia poisoning hypothesis is a core hypothesis recognized domestically and internationally, and ammonia is considered the main pathological factor of all types of HE2. HE patients are often accompanied by hyperammonemia due to the increased production and absorption of ammonia in the intestine of HE patients and the reduced ability to clear ammonia3. Because of its small molecular weight, ammonia is extremely easy to cross the BBB and enter the brain tissue. Therefore, the increase of blood ammonia will cause the increase of intracranial ammonia concentration, change the energy metabolism of brain tissue, cause neuron cells edema, inhibit nerve conduction function, and eventually lead to brain edema, cognitive dysfunction and neurological dysfunction4. Current research provides compelling evidence that oxidative stress and inflammatory responses are independent factors in the development of HE and have a synergistic effect with ammonia5. The experimental results demonstrated that antioxidant intervention can also reduce the symptoms of HE and enhanced spatial learning capabilities of HE rats. Treatment in the early comatose phase can also increase the survival rate of rats6,7. Clinical investigations have demonstrated that the onset of HE in cirrhotic patients is accompanied by an increase in inflammatory factors, and the level of inflammation in patients with HE is higher than that in counterparts8. Anti-inflammatory therapeutic interventions can attenuate cerebral edema, reduce the grade of HE, decelerate the progression of HE, improve survival rate, enhance motor and cognitive function, and facilitate the restoration of learning and memory capacities9. Hyperammonemia can initiate apoptotic pathways in experimental models of HE, and activation of oxidative stress and inflammation can also trigger pathophysiological events in the central nervous system that programmed cell death of neural cells1,10. These findings collectively demonstrate that oxidative stress, inflammation and neuronal apoptosis have synergistic effects with ammonia, which jointly promote the initiation and progression of HE3.
Extensive preclinical and clinical studies have confirmed that hydrogen has anti-oxidation, anti-inflammation, anti-allergy and anti-apoptosis therapeutic properties, and can regulate the autophagic processes, while hydrogen-rich water and hydrogen have the same biological effects11,12. Hydrogen-rich water (HRW) has protective effects on organ ischemia-reperfusion injury, auditory neuropathy, type 2 diabetes, metabolic syndrome and other diseases13,14,15,16. HRW can improve the liver function of patients with hepatitis B by alleviating oxidative stress, alleviate non-alcoholic fatty liver disease in mice by antioxidant and anti-inflammatory effects, attenuate liver injury caused by obstructive jaundice, significantly reduce the incidence of hepatocarcinogenesis, reduce the maximum tumor volume, and also have protective effects on encephalopathy, liver ischemia reperfusion and liver resection injury17,18,19,20,21,22. Lactulose is a first-line therapeutic agent in the clinical treatment of HE, which can reduce the production and absorption of ammonia in the intestine. Recent investigations have revealed that lactulose can be decomposed by bacteria in the intestine, produce a large amount of hydrogen, and subsequently attenuate the level of oxidative stress in the body, which is an indirect antioxidant23. Lactulose treatment in rats undergoing partial hepatectomy can enhance the production of endogenous hydrogen, attenuate oxidative stress and excessive inflammation in the liver, and promote liver regeneration19. These findings suggest that the increase of endogenous hydrogen in lactulose may be one of the mechanisms of its protective effect on HE, and HRW is full of hydrogen.
Emerging evidence indicates that oxidative stress, neuroinflammation, and neuronal apoptosis exhibit synergistic interactions with ammonia toxicity, collectively driving the pathogenesis and progression of HE. Notably, HRW has demonstrated multifaceted therapeutic properties, including potent anti-inflammatory, antioxidant, and anti-apoptotic activities, with established protective effects against both hepatic and neurological disorders. Based on these mechanistic insights and therapeutic profiles, we hypothesize that HRW may exert significant protective effects against the development and progression of HE.
TAA exhibits selective hepatotoxicity with a dose-dependent correlation to hepatic dysfunction. The resulting hepatic pathology closely mimics the characteristic features observed in cirrhotic patients. Based on its pathophysiological relevance, TAA-induced liver injury has been formally recommended by the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) as a validated experimental model for studying HE24. The dose of 900 mg/Kg can induce all stages of symptoms including coma in rats, and is the recommended dose for studying HE25. In this study, we established a HE rat model through intraperitoneal administration of TAA at 900 mg/kg. Comprehensive assessments were conducted, including: (1) monitoring of general physiological status and behavioral alterations, (2) evaluation of hepatic function and plasma ammonia levels, and (3) histopathological examination of liver and brain tissues. These investigations aimed to determine the potential preventive and therapeutic efficacy of HRW against HE. To elucidate the underlying mechanisms, we systematically analyzed oxidative stress markers, inflammatory mediators, and neuronal apoptosis in plasma, prefrontal cortex, and hippocampal tissues. Our findings provide substantial experimental evidence and theoretical foundation for the development of novel therapeutic strategies targeting HE pathogenesis.
Results
Effect of hydrogen-rich water on the results of Morris water maze in rats
Positioning navigation test was used to evaluate spatial learning ability of rats. During the positioning navigation training: with the increase of training times, the station seeking latency of rats in each group was gradually shortened (Fig. 1A). On the 6th day of the experiment, the search latency of T group was significantly longer than that of N group (P < 0.01), and that of H5 group and H10 was significantly shorter than that of T group (P < 0.05 or P < 0.01) (Fig. 1B).
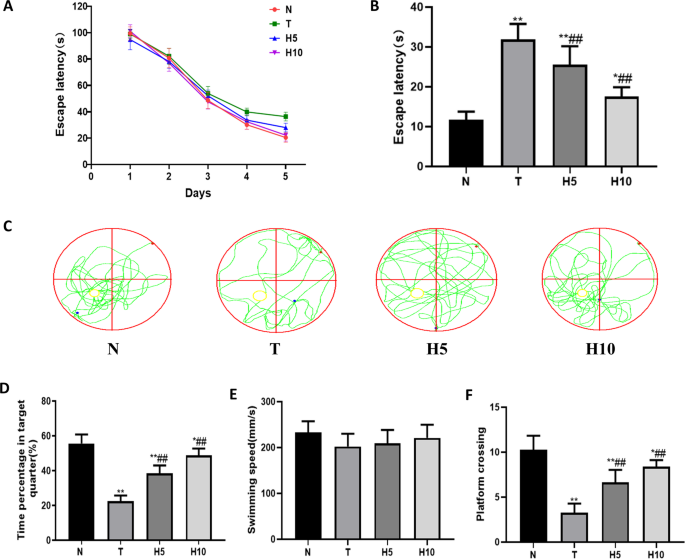
The swimming track, swimming speed and the number of crossing platform were used to evaluate the spatial memory ability of rats in space exploration experiment. On the 6th day of space exploration experiment (Fig. 1E), there was no statistical difference in swimming speed among all groups (P > 0.05). Compared with N group, the percentage of time spent in the target quadrant and the frequency of crossing the platform of rats in T group were significantly decreased (P < 0.01), and were significantly increased after been given HRW (P < 0.01) (Figure C–F).
Effects of hydrogen-rich water on general state observation, neurological score and opening experiment of rats
The rats in N group demonstrated consistent weight gain, maintained normal mental alertness, displayed active movement patterns, exhibited glossy fur, and produced well-formed stool. In contrast, The rats in T group showed significant weight loss, reduced food consumption, decreased spontaneous activity progressing to lethargy and eventual coma, along with dull or shedding fur and watery stool. The changes of rats in H5 group were alleviated compared with those in T group, but they were less energetic, lethargic, less exercise, less hair gloss and less stool. The rats in H10 group had no obvious weight loss, food intake was close to normal, mental performance was slightly poor, activity was less, hair was dull, stool was wet and soft.
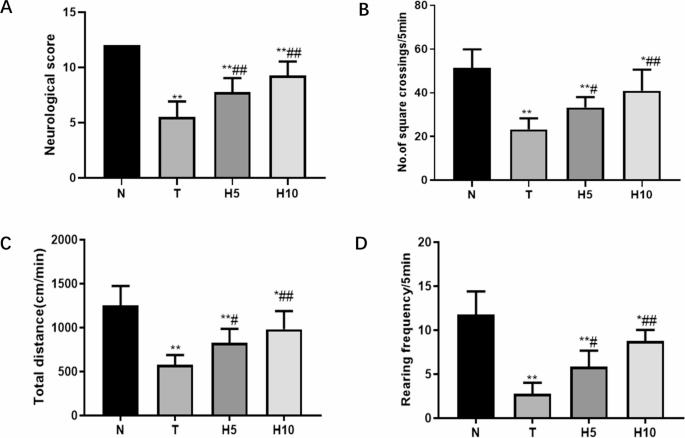
Neurological scores in T group were significantly decreased compared with N group (P < 0.01), and those in H5 group and H10 group were significantly increased compared with T group (P < 0.01) (Fig. 2A). The opening experiment was used to evaluate the spontaneous motor ability of rats in each group, and the span times, total movement distance and upright times of rats in T group were significantly reduced compared with those in N group (P < 0.01). H5 group and H10 group were significantly increased compared with T group (P < 0.05 or P < 0.01) (Fig. 2B–D).
Hydrogen-rich water reduced Amm levels and liver function damage in HE rats
The effects of HRW on plasma Amm, TBIL, ALT and AST levels: The plasma Amm, TBIL, ALT and AST levels in T group were significantly increased compared with those in N group (P < 0.01); H5 and H10 groups were significantly lower than those in T group (P < 0.01) (Fig. 3).

Hydrogen-rich water alleviated liver tissue damage in HE rats
The liver size of rats in N group was normal and the surface was smooth. In T group, the liver was enlarged, the edge was blunt, the surface had a coarse granular feeling, lack of luster, and necrosis was visible. The changes in H5 and H10 groups were less severe than those in T group (Fig. 4A).

Microscopic observation showed that the structure of hepatic lobules in group N was clear, the hepatic cords were arranged neatly, and there was no degeneration and necrosis of hepatic cells and no inflammatory cell infiltration. In T group, fibrous tissue hyperplasia occurred, normal liver tissue was destroyed and wrapped to form clumps, with structural changes similar to liver cirrhosis. The structure of liver lobules could not be distinguished, liver cords were disorganized, hepatocytes were edematous, plasma was pale and loose, necrosis appeared in some places, and a large number of inflammatory cells could be seen. H5 group could distinguish the structure of hepatic lobules, had mild fibrous tissue hyperplasia, inflammatory cell infiltration was less than T group, and the degree of cell degeneration and necrosis was less. The liver lobular structure of H10 group was basically normal, hepatocytes edema, a few showed balloon-like transformation, a small amount of inflammatory cell infiltration, no necrosis (Fig. 4B; Table 1).Table 1 The degree of liver lesions under light microscope
Histomorphological changes of prefrontal cortex and CA1 region of hippocampus in HE rats induced by hydrogen-rich water
Prefrontal cortex: The prefrontal cortex of N group had clear structure and normal neuronal morphology; the structure of the prefrontal cortex in T group was obviously changed, the hierarchy was not clear, the shape of most neurons was irregular, the nuclei showed contraction, the staining was deepened, and the envelope was lightly stained; the prefrontal cortex structure and neuronal pathological changes of H5 and H10 groups were significantly improved compared with those of T group. The number of normal neurons in T group was significantly decreased compared with N group (P < 0.01), and the number of normal neurons in H5 group and H10 group was significantly increased compared with T group (P < 0.01) (Fig. 5A, B).

Hippocampal CA1 region: N group hippocampal neurons were arranged neatly with large and round nuclei; the arrangement of hippocampal neurons in T group was loose, the structure was disordered, some neurons were pyretic and deeply stained, and the number of normal neurons was significantly decreased compared with that in N group (P < 0.01); the pathological changes of neurons in H5 group and H10 group were significantly improved compared with T group, and the number of normal neurons was significantly increased compared with T group (P < 0.01) (Fig. 5A、C).
Hydrogen-rich water reduced the level of oxidative stress in HE rats
The activities of GPx and total superoxide dismutase (T-SOD) in plasma, prefrontal cortex and hippocampus of T group were significantly decreased compared with that of N group (P < 0.01), and those of H5 and H10 groups were significantly increased compared with that of T group (P < 0.01). The MDA content in plasma, prefrontal cortex and hippocampus of T group was significantly increased compared with that of N group (P < 0.01), and the MDA content in H5 and H10 groups was significantly decreased compared with that of T group (P < 0.05 or P < 0.01) (Fig. 6).

Hydrogen-rich water alleviated the inflammatory response in HE rats
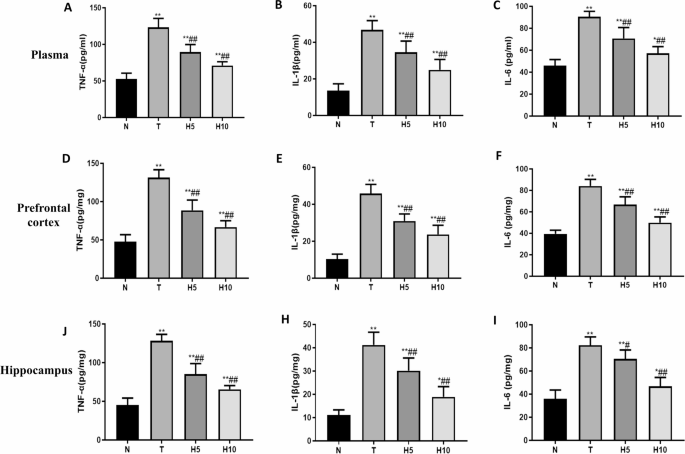
The contents of TNF-α, IL-1β and IL-6 in plasma, prefrontal cortex and hippocampus of T group were significantly increased compared with that of N group (P < 0.01), and the contents of H5 and H10 groups were significantly decreased compared with that of T group (P < 0.01)(Fig. 7).
Hydrogen-rich water alleviated the apoptosis of neurons in HE rats
Compared with N group, the number of neuronal apoptosis in the prefrontal cortex and CA1 region of hippocampus in T group was significantly increased, and the number in H5 group and H10 group was significantly decreased compared with T group. The apoptosis index of T group was significantly increased compared with N group (P < 0.01), and H5 group and H10 group were significantly decreased compared with T group (P < 0.01) (Fig. 8).

Discussion
Hepatic encephalopathy is a neuropsychiatric syndrome frequently associated with acute and chronic liver failure, indicating a state of deterioration of the disease. Ammonia is believed to be a central factor in the pathogenesis of HE, and ammonia entering the liver through the portal vein is predominantly metabolized to urea and glutamine through the urea cycle. As liver disease progresses, urea synthesis in the liver decreases, resulting in compromised ammonia metabolism, leading to hyperammonemia5. Ammonia can easily cross the BBB and cause an increase in intracranial ammonia concentration, which is reduced by glutamine synthetase in astrocytes4. However, glutamine is a osmotically active substance, which can cause neuronal edema and disrupt neuronal metabolic homeostasis. When the concentration of ammonia increases, glutamic acid synthesis increases, and glutamic acid and ammonia synthesis glutamine increase, which will affect the energy metabolism process of cells and consume ATP, and the activity of Na+-K+ -ATPase is inhibited by ammonia, ATP production is reduced, energy source of the brain is reduced, and the body will eventually appear coma26. Elevated cerebral ammonia concentrations breaks the balance between excitatory neurotransmitter glutamate and inhibitory neurotransmitter GABA, causing neuropsychiatric disturbances27.
TAA is a selective hepatotoxic chemical that can induce hepatocellular necrosis and hepatic dysfunction in a dose-dependent manner. TAA can cause elevated blood ammonia, neuronal edema, intracranial oxidative stress, inflammation and neurological impairment. The liver injury induced by TAA is similar to that in cirrhotic patients, and has been widely used in the study of the pathological mechanism of HE25. Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin (TBIL) levels are the most commonly used indicators to test liver function. In this experiment, the spatial learning ability of rats was evaluated by measuring the latency period of station seeking, the spatial memory ability of rats was evaluated by measuring the penetration index, the spontaneous motor ability of rats was evaluated by measuring the total distance of movement and the number of times of crossing the platform, and the exploration ability of rats was evaluated by measuring the number of upright times. Plasma levels of AST, ALT and TBIL were measured to assess liver function. In accordance with the methods described in the literature28, the rat model of HE was established by intrabitoneal injection of TAA 300 mg/Kg/day for 3 consecutive days. It was observed that the rats in the model group showed deterioration of mental state, loss of appetite, weight loss, lethargy, ataxia, coma and other symptoms, increased blood ammonia, decreased liver function, and necrosis of liver tissue. The pathological sections showed the disorder of liver tissue structure, degeneration and necrosis of liver cells, accompanied by neuronal injury, learning memory and spontaneous motor decline, which proved that we successfully established the HE model.
In this study, we observed hepatomegaly with visible necrotic foci on the hepatic surface in HE rat models. This finding contrasts with the characteristic hepatic atrophy typically observed in clinical cases of liver cirrhosis. The observed hepatomegaly may be attributed to the following pathophysiological mechanisms: (1) TAA metabolism generates substantial reactive oxygen species, inducing oxidative stress that damages hepatocyte membranes and organelles, leading to cellular edema; (2) subsequent inflammatory responses contribute to tissue edema; (3) portal venous flow obstruction results in hepatic congestion; and (4) sinusoidal endothelial cell injury accompanied by microthrombus formation causes sinusoidal congestion, collectively contributing to increased liver volume29,30.The hepatic tissue may develop focal necrotic areas due to either ischemic injury or the direct cytotoxic effects of TAA. This pathological manifestation could result from compromised microcirculation leading to cellular hypoxia or through TAA-induced metabolic disturbances that ultimately trigger hepatocyte death31.
HRW can release hydrogen in the body, and hydrogen has the characteristics of small molecular weight and strong permeability, can quickly diffuse to important organelles, and can quickly cross the BBB to brain tissue. Hydrogen does not have toxic side effects on the body, does not affect normal physiological parameters, nor does it affect arterial oxygen saturation or hemodynamics32. In clinical experiments, it was found that oral HRW could reduce the activity of ALT and AST, increase the activity of gamma-glutathione transferase, and increase the content of TBIL, but the changes remained within physiologically normal ranges15. Hydrogen is an ideal antioxidant that can enhance the activity of antioxidant enzymes and has selective antioxidant ability. It can eliminate strong oxidizing substances such as peroxynitrite (ONOO-) that are harmful to the body, while having no effect on weak oxidizing substances such as hydrogen peroxide (H2O2) that have normal physiological functions33,34. HRW has a good anti-inflammatory effect, can effectively inhibit inflammatory cells infiltration, reduce the production of pro-inflammatory factors, reduce inflammation. HRW also has a significant anti-apoptotic effect and can regulate the expression of pro-apoptotic factors and anti-apoptotic factors35. In this experiment, we observed that HRW treatment can ameliorate the symptoms of neuropsychiatric abnormalities in HE rats, improve neurological scores, reduce blood ammonia, alleviate liver and brain tissue damage, improve spatial learning and memory ability of HE rats, and enhance the ability of spontaneous movement and exploration of HE rats. To explore the possible mechanism of hydrogen-rich water alleviating injury in HE rats, we examined oxidative stress, inflammatory response and neuronal apoptosis in plasma, prefrontal cortex and hippocampus.
In recent years, there is sufficient evidence that oxidative stress and inflammation are also important pathological mechanisms of HE, and ammonia jointly promote the occurrence and development of HE. Studies have shown that HE can cause central nervous system damage through oxidative stress, neuronal inflammation and apoptosis1. The prefrontal cortex has the functions of memory, analysis, judgment, thinking and executive control, and plays a very prominent role in behavior, consciousness and cognition. The hippocampus is mainly responsible for memory and learning, and is closely related to mood and sleep wakefulness. The patients with hepatic encephalopathy showed abnormal mood, memory impairment, cognitive function decline, learning and memory ability decrease and sleep disturbance, which may be related to the damage of prefrontal cortex and hippocampus36. The brain is one of the most vulnerable organs to oxidative damage due to its high metabolic rate, the need to consume a lot of oxygen, and the fact that brain tissue is rich in lipids. Malondialdehyde (MDA) is the most stable product of lipid peroxidation and represents the level of oxidative stress in the body. In order to prevent damage to reactive oxygen species (ROS), the brain must activate the antioxidant defense system, and antioxidant enzymes play a key role, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), whose activity reflects the body’s ability to remove oxygen free radicals37. When the liver function is normal, the simple increase of blood ammonia can reduce the activity of antioxidant enzymes in the cerebral cortex and cerebellum of rats, and induce oxidative stress injury38. Furthermore, elevated ammonia concentrations induce cytotoxic edema in astrocytes, initiating a cascade of oxidative stress responses within the brain parenchyma that ultimately lead to neuronal dysfunction and neurological impairment1. Both astrocytic edema and inflammatory mediators can activate N-methyl-D-aspartate (NMDA) receptors, which can reduce the activity of antioxidant enzymes, weaken the body’s antioxidant capacity, and cause neuronal damage and brain cell apoptosis39,40,41. A self-perpetuating pathological cycle is formed between astrocyte edema and oxidative stress response: astrocyte edema causes glutamate accumulation between neurons, and glutamate increases neuronal Ca2 + influx through activation of ionotropic glutamate receptors (NMDA), resulting in changes in mitochondrial osmotic pressure, and ultimately an increase in ROS. NMDA receptor activation and oxidative stress response can aggravate astrocyte swelling3,42,43. In this part of the experiment, we observed that compared with the normal group, the activities of GPx and T-SOD in plasma, prefrontal cortex and hippocampal tissue of rats in the model group were reduced, and the content of MDA was significantly increased, indicating that the oxidative stress level of HE rats increased, which was consistent with previous research results. However, the activities of GPx and T-SOD in plasma, prefrontal cortex and hippocampal tissue of rats were increased, while the content of MDA was significantly decreased after the administration of HRW, indicating that hydrogen-rich water can improve the activity of antioxidant enzymes in HE rats, inhibit lipid peroxidation, and alleviate the oxidative damage induced by TAA.
Systemic inflammatory response accelerates the progression of HE in patients with acute liver failure and can increase the stage of HE, indicating that inflammation is closely related to HE44. In this part of the experiment, we observed that compared with the normal group, the levels of tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) in plasma, prefrontal cortex and hippocampal tissue of rats in the model group were significantly increased, indicating that the level of inflammation in HE rats was significantly increased, and hydrogen-rich water could significantly reduce the level of pro-inflammatory factors in HE rats and alleviate inflammatory damage. We observed that HE rats showed neuronal injury, learning and memory decline and spontaneous motor ability, and measured that the levels of TNF-α, IL-1β and IL-6 in plasma, prefrontal cortex and hippocampal tissue of HE rats were significantly increased, while treatment with HRW alleviated the symptoms of neuropsychiatric abnormalities and brain tissue injury of HE rats. The cognitive ability of HE rats was improved, and the levels of proinflammatory factors in plasma, prefrontal cortex and hippocampus were significantly reduced. We speculated that the reduction of TNF-α, IL-1β and IL-6 and other inflammatory factors may be related to the alleviation of cognitive dysfunction in HE rats. Inflammation is an important pathogenic feature of HE-induced brain injury. High levels of ammonia and free radicals can induce inflammatory genes and subsequently produce various inflammatory mediators. TNF-α, IL-1β, and IL-6, as the most important pro-inflammatory cytokines, are elevated in hepatic encephalopathy and trigger harmful inflammatory responses in the central nervous system. In HE patients and animals, elevated brain levels of these cytokines are associated with neuroinflammation and subsequent abnormal cognitive and motor activity45,46. Peripherally generated inflammatory mediators cannot cross the BBB, so they cannot have a direct impact on the brain, but the peripheral immune system can transmit signals to the brain, induce brain tissue to express pro-inflammatory factors, and promote oxidative stress and mitochondrial dysfunction, further aggravating neuroinflammation and neuronal damage tissue47,48. HRW may reduce the levels of TNF-α, IL-1β and IL-6 in HE rats through the following mechanisms, thereby alleviating the cognitive dysfunction of HE rats: (1) Antioxidant effect: Molecular hydrogen in HRW has a strong antioxidant capacity, which can clear excess ROS and inhibit the activation of pro-inflammatory signaling pathways such as nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) cascades, thus reducing the production of inflammatory factors, and the reduction of inflammatory factors can reduce the apoptosis and necrosis of neurons. Protect brain regions related to learning and memory such as hippocampus and prefrontal cortex49. (2) Inhibition of microglia activation: Molecular hydrogen can inhibit the overactivation of microglia and reduce the release of TNF-α, IL-1β and IL-6, while the reduction of TNF-α and IL-1β can alleviate glutamate excitotoxicity, promote synaptic plasticity and long-term enhancement (LTP), and thus improve learning and memory ability50. (3) Protecting the BBB: By alleviating oxidative stress and inflammatory response, HRW may help maintain the integrity of the BBB and prevent peripheral inflammatory factors from entering the central nervous system (CNS). The reduction of inflammatory factors can improve the brain microenvironment and promote the repair and functional recovery of neurons48,51. Studies have shown that the increased levels of TNF-α, IL-1β and IL-6 in HE patients and animal models are closely related to cognitive dysfunction. Studies have shown that the plasma levels of TNF-α and IL-6 in HE patients are negatively correlated with cognitive function scores52. These findings are consistent with our findings that reducing inflammatory factors is associated with improved cognitive function.
Activation of oxidative stress and inflammation can initiate a series of pathophysiological events in the CNS and promote apoptosis of brain cells10. Apoptosis plays an important role in the pathophysiology of TAA-induced HE. Previous studies have shown that TAA induces up-regulation of caspase-3 and Fas expression in liver tissue, suggesting that TAA has pro-apoptotic activity53. It has also been reported that hyperammonemia can activate apoptotic pathways in experimental HE models1. We chose to establish a rat model of HE by intrabitoneal injection of 300 mg/Kg/day of TAA for 3 consecutive days to simulate the pathophysiological events associated with human HE. Our study showed that after TAA injection, brain tissue ammonia levels increased, GPx and T-SOD levels decreased, and MDA increased, indicating overproduction of ROS and lipid peroxidation in brain cells. This eventually raises the level of pro-inflammatory cytokines, which leads to apoptosis54. Previous studies have confirmed that oxidative cellular damage plays a direct role in the activation of apoptosis, and the overproduction of oxidized species leads to mitochondrial dysfunction, including the loss of mitochondrial membrane potential, the release of cytochrome c from mitochondria to cytoplasm, and the activation of caspase-3 and apoptosis55. Hematoxylin and eosin (H&E) staining showed that in the normal group, the prefrontal cortex and hippocampus were clearly structured, the neurons were in normal shape and arranged neatly, and the nuclei were large and round. After TAA was given, the structure of the prefrontal cortex was significantly changed, and the hierarchy was not clear. Most neurons in the prefrontal cortex and hippocampus were irregular in shape, the nuclei showed shrinkage and deep staining, and the count of normal neurons was significantly reduced. The structural and neuronal pathological changes of frontal cortex and hippocampus were improved to varying degrees after the administration of HRW, and the number of normal neurons increased significantly. The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay has strong sensitivity and specificity and is the preferred method for detecting apoptosis. Our TUNEL staining results showed that the apoptotic death of the brain cells in HE rats increased, and the number of apoptotic neurons in the frontal cortex and hippocampus CA1 region decreased significantly and the apoptotic index decreased after the administration of hydrogen-rich water. Therefore, our data showed that HRW has an anti-apoptotic effect in TAA-induced rat brain tissue.
As summarized in Fig. 9, our study demonstrates that treatment with HRW may protect against TAA-induced HE in rats through antioxidant, anti-inflammatory, and anti-apoptotic mechanisms, offering new insights for therapeutic development. However, its specific mechanism and clinical application need further study. While our study provides valuable insights, several limitations should be acknowledged: (1) The current investigation did not elucidate the molecular mechanisms underlying the protective effects of HRW against HE. Future studies should focus on identifying specific signaling pathways and molecular targets involved in HRW-mediated neuroprotection and hepatoprotection. (2) Our findings in rodent models may have limited translational relevance due to inherent physiological and metabolic differences between rats and humans. Additionally, clinical HE patients often present with multiple comorbidities that may influence therapeutic outcomes. We recommend subsequent investigations in higher mammalian models with closer hepatic physiology to humans, followed by early-phase clinical trials. (3) The study was limited to evaluating short-term effects of HRW on learning and motor functions in HE rats. Comprehensive assessment of long-term outcomes, including cognitive recovery and survival benefits, warrants extended treatment durations and observation periods in future research. (4) Although our data demonstrate the efficacy of HRW monotherapy in attenuating HE pathology in rats, clinical HE management typically involves lactulose and/or rifaximin as first-line therapies. The potential synergistic effects of HRW combined with standard clinical regimens remain to be investigated, which could potentially enhance therapeutic benefits for HE patients. These limitations highlight important directions for future research to further validate and optimize HRW-based therapeutic strategies for HE management.

Materials and methods
Feeding and grouping of animals
SPF-grade healthy adult male Sprague-Dawley (SD) rats weighing 220–250 g were purchased from the Experimental Animal Center of Southwest Medical University. They ate and drank water, exposed to light with normal circadian rhythm, indoor temperature was 22–24℃, and relative humidity was 40-60%. The animal manipulation involved in this experiment was approved by the Ethics Committee of Southwest Medical University and complied with the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication, 8th edition, 2011). The study was conducted following the ARRIVE guidelines (PLoS Bio 8(6), e1000412,2010).
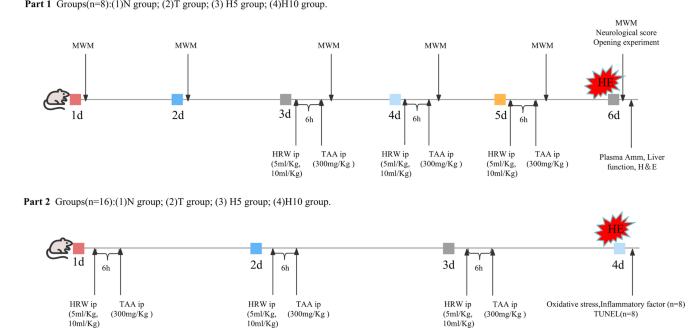
After feeding for one week, a total of 96 rats were randomly divided into 4 groups (n = 24): (1) normal group (N group); (2) Model group (T group); (3) hydrogen-rich water low-dose group (HRW 5 ml/Kg, H5 group); (4) hydrogen-rich water high-dose group (HRW 10 ml/kg, H10 group). When the rats died or were unable to complete behavioral testing, the rats were re-supplemented. The grouping and experimental procedures are detailed in the experimental flow diagram (Fig. 10).
Establishment and administration of the HE rat mode
Except N group, the other groups were intraperitoneally injected with 300 mg/Kg/day of TAA (Shanghai Yuanye Biotechnology Co., LTD., China), and HE rat models were established for 3 consecutive days28. N Group was intraperitoneally injected with equivalent volumes 0.9% NS, and H5 group and H10 group were intraperitoneally injected with HRW (Beijing Vitality Hydrogen Source Beverage Co., LTD., China) 5 ml/Kg and 10 ml/Kg, respectively, 6 h prior to TAA administration.
Animal behavior testing
Morris water maze (MWM)
The Morris water maze (MWM) test was conducted to assess spatial learning and memory capabilities, beginning two days prior to model establishment and continuing for five consecutive days. The maze (Shanghai XinRuan Information Technology Co. Ltd., Shanghai, China) consisted of a round tank (height 50 cm and diameter 150 cm), a platform (diameter 10 cm), and a camera analysis system. In the positioning navigation experiment for five consecutive days (1d-5d), the rats were trained four times a day, each time in a different quadrant of 120 s to find a flooded platform, recording the escape latency, and the rats failed to find the platform within 120 s were manually guided to the platform. The space exploration experiment was carried out on the 6th day, namely, 3 days after modeling, the rats were put into the water facing the pool wall and sailed freely in the water for 120 s to record the escape latency and swimming speed. Then, the platform was withdrawn and the rats were allowed to sail freely in the water again for 120 s, recording the percentage of target quadrants and the number of times they crossed the platform56.
General state observation and neurological score
After the first injection of TAA, the weight, food intake, mental state, hair, frequency of defecation, stool shape, autonomous activity and reflexes of the rats were observed daily. The diagnostic criteria for HE are: reduced autonomic activity, lethargy, delayed response, ataxia, coma and other symptoms in rats57. On the second day after the last injection of TAA, neurological functioning was assessed by measuring the pinna reflex, corneal reflex, tail flexion reflex, escape response reflex, righting reflex, and ataxia, which were assessed and scored on a scale of 0 (no reflex) to 2 (intact reflex). The neurological score at each time point was defined as the summation of these reflex scores58.
Opening experiment
The opening test is often used to assess an animal’s ability to move spontaneously and explore59. On the second day after the last TAA injection, the rats underwent the opening experiment (n = 8). The opening test box is an uncovered wooden box with a black inner wall and a blue bottom plate, and the bottom plate is divided into 25 squares. The volume of the test box is 1 m×1 m× 0.5 m. Keep the laboratory quiet during testing, control the indoor lighting to be dim, and keep the central area of the opening bright. The rat was placed in the center of the experiment box, and the rat was released at the beginning of the experiment. The number of span times (both hind legs crossed the white line), the total distance of spontaneous movement and the number of upright times (both front legs raised) of the rat were recorded. The experimental duration was set at 5 min.
Specimen collection
On the second day after the last TAA injection, the rats fasted for 12 h and drank freely. Rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium (50 mg/kg). After laparotomy, 5 ml of blood was collected by puncture of the inferior vena cava with a blood collection needle, and the plasma was left for 2 h and centrifuged at 3000 r/min for 10 min. The separated plasma was divided and stored at -80℃ for detection of plasma Amm and liver function.
Rats were humanely euthanized via intraperitoneal administration of an overdose of pentobarbital sodium (200 mg/kg). Eight rats were perfused through the left cardiac apex with 150 ml of pre-cooled normal saline (NS), followed by perfusion with 200 ml of 4% paraformaldehyde. After fixation for 30 min, the right lobe of the liver was cut off. The whole brain tissue was extracted from the severed head, and the prefrontal cortex and hippocampal tissues were separated on the ice. The prefrontal cortex and hippocampal tissues were placed in 4% paraformaldehyde, respectively, and fixed for 24 h for Hematoxylin-eosin staining. Sixteen rats were irrigated with 300 ml of pre-cooled NS, and the whole brain tissue was extracted from the rats on the ice. Eight complete brain tissues from each group were stored at -80℃ for TUNEL staining. Eight complete brain tissues from each group were separated from the prefrontal cortex and hippocampal tissues on the ice, and stored at -80℃ after packaging, which was used to make tissue homogenates to measure the expression levels of inflammatory factors and oxidative stress.
The content of amm and TBIL and the activity of ALT and AST were detected
The plasma after centrifugation was obtained, and the serum ammonia (Amm) and TBIL contents and the activity of alanine aminotransferase ALT and aspartate aminotransferase AST were detected with a specific detection kit (Nanjing Jiengcheng Bioengineering Institute, China) according to the manufacturer’s instructions.
Hematoxylin-eosin staining
The liver was fixed with paraformaldehyde 24 h later, and the same part of the right lobe of the liver was cut, and the prefrontal cortex and hippocampus tissues of the brain were then dehydrated, paraffin embedded, and H&E staining was performed after section. Pathological changes of liver, prefrontal cortex and CA1 region of hippocampus were observed under light microscope. The grading was performed by pathologists blinded to the group allocation based on the severity of hepatic pathological changes57. Three high-power visual fields (×400) were randomly selected from each prefrontal cortex section. The number of normal neurons was counted and the average value was obtained. 3 slices of each hippocampal tissue were cut continuously. Under the ×400 microscope, the number of normal neurons in CA1 region was counted and the average value was obtained.
Determination of oxidative stress levels
GPx, T-SOD activity and MDA content of plasma and brain tissue homogenates were measured according to manufacturer’s instructions using a specific detection kit (Nanjing Institute of Biological Engineering, China).
Determination of inflammatory factor levels
The levels of tumor necrosis TNF-α, IL-6 and IL-1β in plasma and brain tissue homogenate were determined by Enzyme linked immunosorbent assay (ELISA) kit (Shanghai Qiaodu Biotechnology Co., LTD., China).
TUNEL staining
TUNEL assay has strong sensitivity and specificity and is the preferred method for detecting apoptosis. The prefrontal cortex and hippocampus tissues of the brain after gradient sucrose dehydration were detected by in situ cell death detection kit (Roche, Switzerland), sliced, fixed, permeable, sealed, reacted, 4’,6-diamidino-2-phenylindole (DAPI) stained, and sealed. The film was sealed and observed under fluorescence microscope. Three high-power visual fields (×200) were randomly selected from each prefrontal cortex section. The number of apoptotic cells and the total number of cells were counted and the average value was obtained. 3 slices of each hippocampal tissue were cut continuously. The number of apoptotic cells in CA1 region and the total number of cells were counted under the ×200 microscope at high power, and the average value was obtained. To calculate the Apoptosis Index (AI), AI = number of apoptotic cells/total number of cells ×100%.
Statistical analysis
All measurement data in this experiment were expressed as mean ± standard deviation (mean ± SD), and data were analyzed using GraphPad Prism 8.0.1 and SPSS 21.0 statistical software. Data met parametric assumptions, as confirmed by the Shapiro-Wilk test (for normality) and Levene’s test (for homogeneity of variances). Subsequently, a one-way ANOVA was performed, followed by Tukey’s HSD test for post hoc pairwise comparisons. P < 0.05 indicated that the difference was statistically significant.
Data availability
Data is provided within the manuscript.
References
- Amirshahrokhi, K. & Niapour, A. Carvedilol attenuates brain damage in mice with hepatic encephalopathy [J]. Int. Immunopharmacol. 111, 109119 (2022).Article CAS PubMed Google Scholar
- Patidar, K. R. & Bajaj, J. S. Covert and overt hepatic encephalopathy: diagnosis and management [J]. Clin. Gastroenterol. Hepatol.. 13 (12), 2048–2061 (2015).Article PubMed PubMed Central Google Scholar
- Saleh, D. O., Mansour, D. F. & Fayez, A. M. Thioacetamide-induced acute hepatic encephalopathy: central vs peripheral effect of allicin [J]. Metab. Brain Dis. 36 (6), 1331–1340 (2021).Article CAS PubMed Google Scholar
- Vidal-Cevallos, P., Chávez-Tapia, N. C. & Uribe, M. Current approaches to hepatic encephalopathy [J]. Ann. Hepatol. 27 (6), 100757 (2022).Article CAS PubMed Google Scholar
- Kwon, K. W. et al. Hepatoprotective effect of sodium hydrosulfide on hepatic encephalopathy in rats [J]. Korean J. Physiol. Pharmacol. 23 (4), 263–270 (2019).Article CAS PubMed PubMed Central Google Scholar
- Chi, D. et al. Role of oxidative/nitrative stress in hepatic encephalopathy induced by thioacetamide [J]. Die Pharm.. 66 (5), 378–381 (2011).CAS Google Scholar
- Wu, Y. B. et al. Artesunate restores spatial learning of rats with hepatic encephalopathy by inhibiting ammonia-induced oxidative damage in neurons and dysfunction of glutamate signaling in astroglial cells [J]. Biomed. Pharmacother. 84, 972–978 (2016).Article CAS PubMed Google Scholar
- Shawcross, D. L. et al. Role of ammonia and inflammation in minimal hepatic encephalopathy [J]. Metab. Brain Dis. 22 (1), 125–138 (2007).Article CAS PubMed Google Scholar
- Agusti, A. et al. p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts [J]. Gut 60 (11), 1572–1579 (2011).Article CAS PubMed Google Scholar
- Amirshahrokhi, K. & Niapour, A. Methylsulfonylmethane protects against ethanol-induced brain injury in mice through the Inhibition of oxidative stress, proinflammatory mediators and apoptotic cell death [J]. Int. Immunopharmacol. 106, 108638 (2022).Article CAS PubMed Google Scholar
- Yang, M. et al. Hydrogen: A novel option in human disease treatment [J]. Oxid. Med. Cell Longev. 2020, 8384742 (2020).
- Ohta, S. Will the hydrogen therapy be approved shortly? [J]. Ann. Transl Med. 8 (6), 264 (2020).Article PubMed PubMed Central Google Scholar
- Wu, M. J. et al. Protective effects of hydrogen rich water on the intestinal ischemia/reperfusion injury due to intestinal intussusception in a rat model [J]. Med. Gas Res. 7 (2), 101–106 (2017).Article CAS PubMed PubMed Central Google Scholar
- Qu, J. et al. Inhalation of hydrogen gas attenuates ouabain-induced auditory neuropathy in gerbils [J]. Acta Pharmacol. Sin. 33 (4), 445–451 (2012).Article CAS PubMed PubMed Central Google Scholar
- Nakao, A. et al. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study [J]. J. Clin. Biochem. Nutr.46 (2), 140–149 (2010).Article MathSciNet PubMed PubMed Central Google Scholar
- Kajiyama, S. et al. Supplementation of hydrogen-rich Water Improves Lipid and Glucose Metabolism in Patients with Type 2 Diabetes or Impaired Glucose Tolerance [J]28137–143 (Nutrition research (New York, NY), 2008). 3.
- Xia, C. et al. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis B [J]. Clin. Transl. Sci. 6 (5), 372–375 (2013).Article CAS PubMed PubMed Central Google Scholar
- Li, S. W. et al. Hydrogen-rich water protects against liver injury in nonalcoholic steatohepatitis through HO-1 enhancement via IL-10 and Sirt 1 signaling [J]. Am. J. Physiol. Gastrointest. Liver Physiol. 320 (4), G450–g463 (2021).Article CAS PubMed Google Scholar
- Yu, J. et al. Lactulose accelerates liver regeneration in rats by inducing hydrogen [J]. J. Surg. Res. 195 (1), 128–135 (2015).Article CAS PubMed Google Scholar
- Kawai, D. et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice [J]. Hepatology 56 (3), 912–921 (2012).Article CAS PubMed Google Scholar
- Chen, G. et al. Hydrogen inhalation is superior to mild hypothermia for improving neurological outcome and survival in a cardiac arrest model of spontaneously hypertensive rat [J]. Shock, (2017).
- Zhang, Q. et al. Comparative study on protective effect of hydrogen rich saline and adipose-derived stem cells on hepatic ischemia-reperfusion and hepatectomy injury in swine [J]. Biomed. Pharmacother. 120, 109453 (2019).Article CAS PubMed Google Scholar
- Chen, X. et al. Lactulose: an effective preventive and therapeutic option for ischemic stroke by production of hydrogen [J]. Med. Gas Res. 2, 3 (2012).Article CAS PubMed PubMed Central Google Scholar
- Butterworth, R. F. et al. Experimental models of hepatic encephalopathy: ISHEN guidelines [J]. Liver Int. 29 (6), 783–788 (2009).Article PubMed Google Scholar
- Mladenović, D. et al. Behavioral and electroencephalographic manifestations of thioacetamide-induced encephalopathy in rats [J]. Can. J. Physiol. Pharmacol. 90 (9), 1219–1227 (2012).Article PubMed Google Scholar
- Nishimaki, K. et al. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment [J]. Curr. Alzheimer Res. 15(5), 482–492 (2018).
- Odeh, M. et al. Serum levels of tumor necrosis factor-alpha correlate with severity of hepatic encephalopathy due to chronic liver failure [J]. Liver Int.. 24 (2), 110–116 (2004).Article CAS PubMed Google Scholar
- Elsherbini, D. M. A. et al. Astrocytes profiling in acute hepatic encephalopathy: possible enrolling of glial fibrillary acidic protein, tumor necrosis factor-alpha, inwardly rectifying potassium channel (Kir 4.1) and aquaporin-4 in rat cerebral cortex [J]. Front. Cell. Neurosci. 16, 896172 (2022).Article CAS PubMed PubMed Central Google Scholar
- Sepehrinezhad, A. et al. Drug-induced-acute liver failure: a critical appraisal of the thioacetamide model for the study of hepatic encephalopathy [J]. Toxicol. Rep. 8, 962–970 (2021).Article CAS PubMed PubMed Central Google Scholar
- Ezhilarasan, D. Molecular mechanisms in thioacetamide-induced acute and chronic liver injury models [J]. Environ. Toxicol. Pharmacol. 99, 104093 (2023).Article CAS PubMed Google Scholar
- Abdelaziz, R. R., Abdelrahman, R. S. & Abdelmageed, M. E. SB332235, a CXCR2 antagonist, ameliorates thioacetamide-induced hepatic encephalopathy through modulation of the PI3K/AKT pathways in rats [J]. Neurotoxicology 92, 110–121 (2022).Article CAS PubMed Google Scholar
- Ono, H. et al. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level [J]. Med. Gas Res. 2 (1), 21 (2012).Article CAS PubMed PubMed Central Google Scholar
- Ohsawa, I. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals [J]. Nat. Med. 13 (6), 688–694 (2007).Article CAS PubMed Google Scholar
- Tian, Y. et al. Effects of Hydrogen-Rich saline on Hepatectomy-Induced postoperative cognitive dysfunction in old mice [J]. Mol. Neurobiol. 54 (4), 2579–2584 (2017).Article CAS PubMed Google Scholar
- Ge, L. et al. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases [J]. Oncotarget 8 (60), 102653–102673 (2017).Article PubMed PubMed Central Google Scholar
- Shao, Y. et al. The pathogenesis of hepatic encephalopathy and its diagnosis and treatment progress [J]. Int. J. Epidemiol. Epidemiol. 44 (3), 194–198 (2017).Google Scholar
- Amirshahrokhi, K. & Abzirakan, A. Carvedilol attenuates acrylamide-induced brain damage through Inhibition of oxidative, inflammatory, and apoptotic mediators [J]. Iran. J. Basic. Med. Sci. 25 (1), 60–67 (2022).PubMed PubMed Central Google Scholar
- Singh, S., Koiri, R. K. & Trigun, S. K. Acute and chronic hyperammonemia modulate antioxidant enzymes differently in cerebral cortex and cerebellum [J]. Neurochem Res.33 (1), 103–113 (2008).Article CAS PubMed Google Scholar
- Gorg, B. et al. Inflammatory cytokines induce protein tyrosine nitration in rat astrocytes [J]. Arch. Biochem. Biophys. 449 (1–2), 104–114 (2006).Article PubMed Google Scholar
- Kosenko, E. et al. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo [J]. Brain Res. 981 (1–2), 193–200 (2003).Article CAS PubMed Google Scholar
- Jiang, W., Desjardins, P. & Butterworth, R. F. Hypothermia attenuates oxidative/nitrosative stress, encephalopathy and brain edema in acute (ischemic) liver failure [J]. Neurochem. Int. 55 (1–3), 124–128 (2009).Article CAS PubMed Google Scholar
- Haussinger, D. & Schliess, F. Pathogenetic mechanisms of hepatic encephalopathy [J]. Gut 57 (8), 1156–1165 (2008).Article CAS PubMed Google Scholar
- Rodriguez-Rodriguez, P., Almeida, A. & Bolaños, J. P. Brain energy metabolism in glutamate-receptor activation and excitotoxicity: role for APC/C-Cdh1 in the balance Glycolysis/pentose phosphate pathway [J]. Neurochem. Int. 62 (5), 750–756 (2013).Article CAS PubMed Google Scholar
- Shawcross, D. L. et al. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis [J]. J. Hepatol. 40(2), 247–254 (2004).Article CAS PubMed Google Scholar
- Ochoa-Sanchez, R. & Rose, C. F. Pathogenesis of hepatic encephalopathy in chronic liver disease [J]. J. Clin. Experimental Hepatol. 8 (3), 262–271 (2018).Article Google Scholar
- Sun, X. et al. Pro-inflammatory cytokines serve as communicating molecules between the liver and brain for hepatic encephalopathy pathogenesis and Lycium barbarum polysaccharides protection [J]. J. Ethnopharmacol. 248, 112357 (2020).Article CAS PubMed Google Scholar
- Seyan, A. S. Changing face of hepatic encephalopathy: role of inflammation and oxidative stress [J]. World J. Gastroenterol. 16 (27), 3347 (2010).Article CAS PubMed PubMed Central Google Scholar
- Cabrera-Pastor, A. et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: underlying mechanisms and therapeutic implications [J]. Acta Physiol. (Oxford, England). 226 (2), e13270 (2019).Article Google Scholar
- Hussien, Y. A. et al. Linagliptin attenuates thioacetamide-induced hepatic encephalopathy in rats: modulation of C/EBP-β and CX3CL1/Fractalkine, neuro-inflammation, oxidative stress and behavioral defects [J]. Life Sci. 295, 120378 (2022).Article CAS PubMed Google Scholar
- Zhuang, X. et al. Molecular hydrogen attenuates sepsis-induced neuroinflammation through regulation of microglia polarization through an mTOR-autophagy-dependent pathway [J]. Int. Immunopharmacol. 81, 106287 (2020).Article CAS PubMed Google Scholar
- Guo, S. X. et al. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation [J]. J. Translational Med. 13, 183 (2015).Article Google Scholar
- Shawcross, D. L. et al. Infection and systemic inflammation, not ammonia, are associated with grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis [J]. J. Hepatol. 54(4), 640–649 (2011).Article CAS PubMed Google Scholar
- Mohamed, E., Hafez, D. J. B. & c m red, therapies. Gallic acid and metformin co-administration reduce oxidative stress, apoptosis and inflammation via Fas/caspase-3 and NF-κB signaling pathways in thioacetamide-induced acute hepatic encephalopathy in rats [J]. 23(1): 265. (2023).
- Hajipour, S. et al. The effects of thymoquinone on memory impairment and inflammation in rats with hepatic encephalopathy induced by thioacetamide. J 36 (5), 991–1002 (2021).CAS Google Scholar
- Sedik, A. et al. Neuromodulatory role of L-arginine: nitric oxide precursor against thioacetamide-induced-hepatic encephalopathy in rats via downregulation of NF-κB-mediated apoptosis. J 30 (35), 84791–84804 (2023).CAS Google Scholar
- Vorhees, C. V. et al. Morris water maze: procedures for assessing spatial and related forms of learning andmemory [J]. Nat Protoc. 1(2), 848–858 (2006).
- Li, X. et al. Intervention of Taohe Chengqi Decoction in the treatment of rat hepatic encephalopathy model [J]. Chin. J. Comp. Med. 26 (10), 14–18 (2016).ADS CAS Google Scholar
- McMillin, M. et al. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline [J]. J. Neuroinflammation. 11, 121 (2014).Article PubMed PubMed Central Google Scholar
- Bloch, J. R., Dawley, K. & Suplee, P. D. Application of the Kessner and Kotelchuck prenatal care adequacy indices in a preterm birth population [J]. Public. Health Nurs. 26(5), 449–459 (2009).Article PubMed Google Scholar
Funding
This study was supported by grants from the Sichuan Science and Technology Program (No.2022YFS0615), Luzhou Science and Technology Program (No.2023SYF099), Zigong Key Science and Technology Program (No.2023NKY0302 and No.2023YLWS16).
Author information
Authors and Affiliations
- Zigong Fourth People’s Hospital, Zigong, 643000, Sichuan, ChinaXujiao Wang & Xiao Liu
- Department of Anesthesiology, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, Sichuan, ChinaPeng Zhou, Jianguo Feng, Jing Jia & Jun Zhou
- Anesthesiology and Critical Care Medicine Key Laboratory of Luzhou, Southwest Medical University, No. 25 Taiping Road, Jiangyang District, Luzhou, 646000, Sichuan, ChinaXujiao Wang, Xiao Liu, Peng Zhou, Jianguo Feng, Jing Jia, Ye Chen & Jun Zhou
- Laboratory of Neurological Diseases and Brain Function, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, ChinaBingqing Xie
- Institute of Epigenetics and Brain Science, Southwest Medical University, Luzhou, 646000, ChinaBingqing Xie
- Department of Traditional Chinese Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, Sichuan, ChinaYe Chen
Contributions
Xujiao Wang, Xiao Liu, and Peng Zhou conducted the experiments, data collection, and analysis, and wrote and edited the manuscript editing. Ye Chen and Bingqing Xie performed experiments and edited the manuscript. Jianguo Feng, and Jing Jia assisted with data interpretation and manuscript editing. Jun Zhou was responsible for study conception and design, data analysis and interpretation, study supervision, and manuscript revision. All authors have read and approved of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Liu, X., Zhou, P. et al. Treatment with hydrogen-rich water protects against thioacetamide-induced hepatic encephalopathy in rats through stabilizing liver–brain disturbance. Sci Rep 15, 17901 (2025). https://doi.org/10.1038/s41598-025-02891-2
- Received12 December 2024
- Accepted16 May 2025
- Published23 May 2025
- DOIhttps://doi.org/10.1038/s41598-025-02891-2
Share this article
Anyone you share the following link with will be able to read this content:Get shareable link
Provided by the Springer Nature SharedIt content-sharing initiative
Keywords
Subjects
- Sections
- Figures
- References
- Abstract
- Introduction
- Results
- Discussion
- Materials and methods
- Data availability
- References
- Funding
- Author information
- Ethics declarations
- Additional information
- Rights and permissions
- About this article
Advertisement
Scientific Reports (Sci Rep)
ISSN 2045-2322 (online)
nature.com sitemap
About Nature Portfolio
Discover content
Publishing policies
Author & Researcher services
- Reprints & permissions
- Research data
- Language editing
- Scientific editing
- Nature Masterclasses
- Research Solutions
Libraries & institutions
Advertising & partnerships
Professional development
Regional websites
- Privacy Policy
- Use of cookies
- Your privacy choices/Manage cookies
- Legal notice
- Accessibility statement
- Terms & Conditions
- Your US state privacy rights
© 2025 Springer Nature LimitedClose
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.Email addressSign upI agree my information will be processed in accordance with the Natureand Springer Nature Limited Privacy Policy.
0 Comments